A Chamber of Secrets: The Gut-Brain Axis and the Immune System
Explore the intricate relationship between the gut-brain axis and the immune system. Learn how the MGB influences overall well-being.

Key Takeaways
- MGB Axis Studies: Research on the Microbiota-Gut-Brain (MGB) axis primarily involves cross-sectional studies linking gut microbes to brain structure, but a deeper understanding of gut-brain communication is needed.
- ENS Similarities with CNS: The Enteric Nervous System (ENS), known as the "second brain," shares traits with the central nervous system (CNS), prompting investigations into how CNS mechanisms affect ENS and vice versa.
- Serotonin's Dual Role: Serotonin, crucial in both CNS and gut, influences the development and function of both systems.
- Interactions of ENS, CNS, and Microbiome: These systems play vital roles in brain and gut development, offering potential therapeutic targets for various medical conditions.
- Remaining Research Questions: Despite impressive progress, critical unanswered questions require further exploration for effective therapies.
- Advancements in Microbiome Study: Recent technology advances and cohort studies have expanded knowledge of the microbiome's impact on health.
- Microbial Variation: Considerable differences exist in microbial composition among individuals, with factors influencing these variations still under study.
- Gut Microbiome and Mental Health: Changes in the gut microbiome are linked to neurological and mental conditions, though causation remains unclear.
- Maternal Influence on Microbiome: Maternal diet and stress during pregnancy can significantly shape an infant's microbiome, affecting development.
- Microbial Influence on Neurodevelopment and ENS: Microbes impact neurodevelopment, including the ENS, with implications for gut motility and neural coding.
Introduction
The gut-brain axis is a complex bidirectional communication system between the gastrointestinal tract and the central nervous system.
Recent research has shown that this connection is not limited to the nervous system, but also involves the immune system and the gut microbiome.
The immune system plays a vital role in maintaining the balance between the gut and brain, and disruptions in this balance have been linked to a range of health issues, including autoimmune disorders, mood disorders, and neurodegenerative diseases.
Understanding the interplay between the immune system and the gut-brain axis is crucial for developing effective prevention and treatment strategies for these conditions.
In this article, we will explore the relationship between the immune system and the gut-brain axis, and how they work together to keep us healthy.
In This Article
The Gut-Brain Axis: How Our Second Brain Keeps Us in Balance
The gut-brain axis is a vital component in maintaining homeostasis within the body.
There are many intrinsic and extrinsic factors that influence signaling along this axis, which in turn modulates the function of both the enteric and central nervous systems.
Recent studies have identified the microbiome as a significant factor in modulating gut-brain signaling, leading to the establishment of the concept of a microbiota-gut-brain axis.
The idea that the brain and gut are linked was known by some of the greatest modern biologists like Darwin, Pavlov, James, Bernard, and Cannon before the 20th century Mayer E.A. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011; 12: 453-466 Scopus (773) PubMed Crossref Google Scholar , but it wasn't until then that scientists began studying how changes in gut physiology can affect emotions. Initially, these studies were limited because of the lack of advanced technology, but recent research has shown that there are actually very close links between mental and gastrointestinal health. Drossman D.A. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016; 150: 1262-1279 Abstract Full Text Full Text PDF Google ScholarUnraveling the Gut-Brain Axis: From Ancient Beliefs to Modern Science
Pioneering Insights in the 19th Century
This research has also revealed some of the mechanisms behind the connection.
The Role of Nerves and Beyond
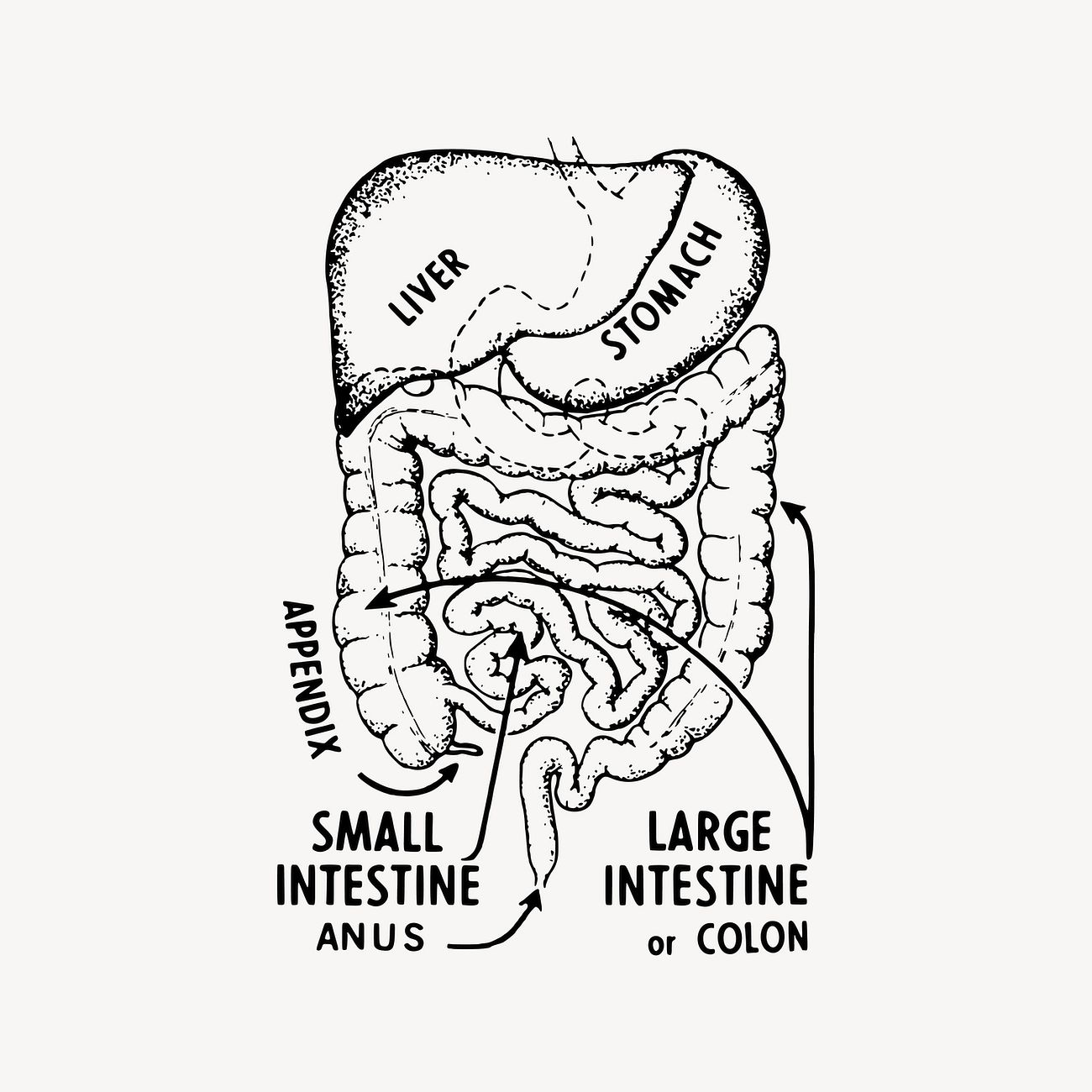
The nerves in our gut play a big role in how it works.
The Enteric Nervous System (ENS) is made up of lots of neurons and glia and it goes all through the intestine.
This system can work without help from the brain, but the brain [6] and other parts of the Autonomic Nervous System (ANS) can also affect how our gut works.
This system can work without help from the brain, but the brain Browning K.N. Travagli R.A. Central control of gastrointestinal motility. Curr Opin Endocrinol Diabetes Obes. 2019; 26: 11-16 Scopus (7) PubMed Crossref Google Scholar and other parts of the Autonomic Nervous System (ANS) can also affect how our gut works.Other things like the immune system and the bacteria living in our gut can also play a role.
Disorders and the Gut-Brain Axis
Studies have shown that there is a link between the brain and the gut, and the way they work together.
For some illnesses, like Parkinson's disease, problems with the gut might start happening before issues with the brain show up.
Other disorders of the brain-gut connection, like irritable bowel syndrome (IBS), can include psychological symptoms and mental health issues. Bove C. Travagli R.A. Neurophysiology of the brain stem in Parkinson's disease. J Neurophysiol. 2019; 121: 1856-1864 Scopus (6) PubMed Crossref Google ScholarHistorical Perspective on Holistic Medicine
As far back as Ancient Greece, philosophers like Hippocrates, Plato, and Aristotle believed that the brain and the rest of the body are connected.
This idea eventually led to the realization that when studying diseases, looking at the whole person is more important than just looking at a single organ system Drossman D.A. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016; 150: 1262-1279 Abstract Full Text Full Text PDF Google ScholarIt wasn't until the 1840s that William Beaumont proved that emotions can have an effect on digestion, showing that the brain and gut are connected and that there is something called a gut-brain axis.
Signs and symptoms in the gut are an important part of illnesses that involve the brain and the digestive system, such as Irritable Bowel Syndrome (IBS).
These are usually connected to emotional issues and mental health diagnoses.
Modern Tools for Understanding
Thanks to brain scans, it is now possible to see how the brain and the gut are linked.
This means that we can see how feelings in the gut can activate areas of the brain that control how we feel emotionally. Mayer E.A. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011; 12: 453-466 Scopus (773) PubMed Crossref Google Scholar The gastrointestinal (GI) system is controlled by a complex network of nerves and muscles Margolis K.G. Gershon M.D. Bogunovic M. Cellular organization of neuroimmune interactions in the gastrointestinal tract. Trends Immunol. 2016; 37: 487-501 Scopus (33) PubMed Abstract Full Text Full Text PDF Google Scholar , Rao M. Gershon M.D. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016; 13: 517-528 Scopus (181) PubMed Crossref Google Scholar This network includes the enteric nervous system (ENS) which is inside the intestines, as well as signals from the brain Browning K.N. Travagli R.A. Central control of gastrointestinal motility. Curr Opin Endocrinol Diabetes Obes. 2019; 26: 11-16 Scopus (7) PubMed Crossref Google Scholar and other parts of the autonomic nervous system.The immune system and the microbiome also have an influence on GI physiology.
It's not just the brain that's in charge- the gut can also send signals to the brain, and changes in how the two systems communicate can lead to health problems.
The Microbiome's Impact
In recent years, scientists have begun to realize how important our microbiome (the trillions of tiny living organisms found in our gut) is in how it affects our brain.
Studies are still relatively new but have already discovered how the microbiome can influence a person's emotions, thinking, and learning.
This has made the microbiome a possible target for therapies in the future. Rhee S.H. Pothoulakis C. Mayer E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009; 6: 306-314 Scopus (646) PubMed Crossref Google Scholar , Collins S.M. Surette M. Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012; 10: 735-742 Scopus (824) PubMed Crossref Google Scholar
The Gut Connectome
Gut microorganisms communicate with the central nervous system (CNS) through various channels, including neural, endocrine, and immune signaling.
Similarly, the CNS can influence the gut microbiota both directly, by inducing the expression of virulence genes through stress mediators, and indirectly, by controlling gut function through the autonomic nervous system (ANS) which regulates factors like motility, immune response, and secretion.
Additionally, the enteric nervous system (ENS) plays a role in modulating microbial composition by affecting secretion, motility, permeability, and immune defense Osadchiy V. Martin C.R. Mayer E.A. Gut microbiome and modulation of CNS function. Compr Physiol. 2019; 10: 57-72 Scopus (7) PubMed Crossref Google Scholar These interconnected pathways are now being recognized as a complex communication network, often referred to as the gut connectome Ye L. Liddle R.A. Gastrointestinal hormones and the gut connectome. Curr Opin Endocrinol Diabetes Obes. 2017; 24: 9-14 PubMed Google ScholarStudies have shown that some gastrointestinal problems in neurological conditions can be caused by genetic defects and environmental factors that affect both gut and brain development.
The enteric nervous system (ENS), also known as the "second brain," has many similarities with the central nervous system (CNS).
These similarities have led researchers to investigate how the mechanisms that cause CNS disorders can also lead to ENS dysfunction and vice versa.
For instance, serotonin, a key transmitter in both the CNS and gut, can affect the development and long-term functions of both systems.
Future Therapeutic Targets
The critical involvement of the enteric nervous system (ENS), central nervous system (CNS), and the microbiome in brain and gut development and function suggests that understanding their relationships can lead to new therapeutic targets for common yet poorly understood medical conditions.
While current research is impressive, there are still important unanswered questions that need to be addressed to facilitate the development of effective therapies.
This review focuses on the current evidence supporting the interactions between the brain, gut, and gut microbiota, and their contribution to human disease.
The Microbiota-Gut-Brain (MGB) Axis
Unraveling the Microbiome: Advances in the Past Decade
The past decade has seen a huge jump in our comprehension of the microbiome and its connection to a variety of aspects of health and disease, thanks to the improvement of next-generation sequencing technologies and large cohort studies Gilbert J.A. Blaser M.J. Caporaso J.G. et al. Current understanding of the human microbiome. Nat Med. 2018; 24: 392-400 Scopus (483) PubMed Crossref Google ScholarMicrobial Diversity in the Gut: Key Insights and Challenges
In humans, the greatest number of microbes is found in the gut, where an estimated 3x1013 microbes from over 60 genera can be found Sender R. Fuchs S. Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016; 14e1002533 PubMed Crossref Google ScholarThere is a huge amount of difference between people when it comes to their microbial makeup and we are still learning about the factors that affect it in both healthy and sick people.
Research studies have also shown that changes in the diversity and proportion of bacteria and their by-products in the gut are linked to many neurological and mental health issues like Parkinson's disease, Alzheimer's disease, autism, and depression Bastiaanssen T.F.S. Cowan C.S.M. Claesson M.J. et al. Making sense of ... the microbiome in psychiatry. Int J Neuropsychopharmacol. 2019; 22: 37-52 Scopus (0) PubMed Crossref Google ScholarThese studies do not yet offer proof that the gut microbiome is the cause of these conditions.
In the field of clinical research, the exploration of the MGB axis has been primarily limited to cross-sectional studies that establish connections between gut microbes and brain structure in both healthy individuals and those with various medical conditions Long-Smith C. O'Riordan K.J. Clarke G. et al. Microbiota-gut-brain axis: new therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2020; 60: 477-502 Scopus (40) PubMed Crossref Google ScholarThere is a big difference in what we know about how the gut and the brain communicate with each other.
Scientists have studied how the immune system, signals from the gut to the brain, and the vagus nerve (which links the gut and the brain) help the gut and the brain to send messages to each other Fülling C. Dinan T.G. Cryan J.F. Gut microbe to brain signaling: what happens in vagus…. Neuron. 2019; 101: 998-1002 Scopus (85) PubMed Abstract Full Text Full Text PDF Google ScholarHowever, more research is needed to really understand the communication between the gut and the brain.
HIGHLIGHT
Advancements in sequencing technology have shown connections between the gut microbiome and neurological/mental health conditions, though causation remains unconfirmed. More research is needed.
MGB Axis in Early Life

Maternal Influence on Infant Microbes
It is still uncertain whether microbial colonization takes place in the womb Perez-Munoz M.E. Arrieta M.C. Ramer-Tait A.E. et al. A critical assessment of the "sterile womb" and "in utero colonization" hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017; 5: 48 Scopus (0) PubMed Crossref Google Scholar Yet, maternal diet Chu D.M. Antony K.M. Ma J. et al. The early infant gutmicrobiome varies in association with a maternal high-fat diet. Genome Med. 2016; 8: 77 Scopus (151) PubMed Crossref Google Scholar and stress during pregnancy Jasarevic E. Howard C.D. Misic A.M. et al. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci Rep. 2017; 7: 44182 Scopus (0) PubMed Crossref Google Scholar have been found to influence an infant's microbiome. The maternal microbiome can thus significantly affect an infant's development and physiology Ganal-Vonarburg S.C. Hornef M.W. Macpherson A.J. Microbial-host molecular exchange and its functional consequences in early mammalian life. Science. 2020; 368: 604-607 Scopus (27) PubMed Crossref Google Scholar Interestingly, the times of greatest changes in the developing microbiota partially match with the periods of development of the brain and digestive system. Cowan C.S.M. Dinan T.G. Cryan J.F. Annual research review: critical windows - the microbiota-gut-brain axis in neurocognitive development. J Child Psychol Psychiatry. 2020; 61: 353-371 Scopus (19) PubMed Crossref Google ScholarThis suggests that the microbiome may be important for these important bodily systems.
Microbial Transformations from Birth to Adulthood
Changes in the microbiota occur continuously during adolescence Stewart C.J. Ajami N.J. O'Brien J.L. et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018; 562: 583-588 Scopus (364) PubMed Crossref Google Scholar , but these modifications become more gradual and move towards an adult-like profile. Yatsunenko T. Rey F.E. Manary M.J. et al. Human gut microbiome viewed across age and geography. Nature. 2012; 486: 222-227 Scopus (3761) PubMed Crossref Google Scholar In adults, what you consume has the biggest effect on the composition of the microbiota Sandhu K.V. Sherwin E. Schellekens H. et al. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res. 2017; 179: 223-244 PubMed Abstract Full Text Full Text PDF Google Scholar , although antibiotic use can also disrupt the microbiota at any age. Willing B.P. Russell S.L. Finlay B.B. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol. 2011; 9: 233-243 Scopus (382) PubMed Crossref Google Scholar After a few days of being born, a shift occurs in the microbiota population in order to provide the nutrients necessary to support a baby's brain and body development . Koenig J.E. Spor A. Scalfone N. et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011; 108: 4578-4585 Scopus (1456) PubMed Crossref Google ScholarThis change in gut microbiota may depend on if the baby is breast or formula-fed.
At weaning, a major change takes place, when an infant transitions from breastmilk or formula to a solid diet, which is observed in various species including humans and rodents . Al Nabhani Z. Dulauroy S. Marques R. et al. A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity. 2019; 50: 1276-1288e5 Scopus (111) PubMed Abstract Full Text Full Text PDF Google Scholar , Yatsunenko T. Rey F.E. Manary M.J. et al. Human gut microbiome viewed across age and geography. Nature. 2012; 486: 222-227 Scopus (3761) PubMed Crossref Google Scholar Although there are continuous modifications until adolescence, these changes are more gradual and directed towards an adult-like profile . Agans R. Rigsbee L. Kenche H. et al. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiology Ecology. 2011; 77: 404-412 Scopus (0) PubMed Crossref Google ScholarDifferences in gut microbiota may be related to how an infant is fed, either through breast milk or formula.
Most studies suggest that when compared to formula-fed babies, breastfed babies have a less diverse and less rich microbiome, with higher levels of Proteobacteria and Bifidobacteria, and lower levels of Bacteroidetes and Firmicutes.
However, these differences don't seem to be connected to differences in the behavior of the babies, such as colic Davis E.C. Dinsmoor A.M. Wang M. et al. Microbiome composition in pediatric populations from birth to adolescence: impact of diet and prebiotic and probiotic interventions. Dig Dis Sci. 2020; 65: 706-722 Scopus (10) PubMed Crossref Google Scholar , Ouald Chaib A. Levy E.I. Ouald Chaib M. et al. The influence of the gastrointestinal microbiome on infant colic. Expert Rev Gastroenterol Hepatol. 2020; 14: 1-13 Scopus (0) PubMed Crossref Google ScholarThe postnatal microbiota is not very stable in the first few months of a baby's life, but it gets more stable and varied as the baby grows older.
How the baby's gut gets colonized when they are born depends on lots of things, like how they were born, if they are breastfed, if they were born prematurely, what the environment is like, their genetics if they have had antibiotics, and things about their mother like if she is ill, stressed or overweight.
However, these differences do not seem to be related to infant behavior like colic.
HIGHLIGHT
Maternal diet and stress affect infant microbiome, impacting brain and digestive system development. Microbiota changes from birth to adulthood, influenced by diet and antibiotics.
Microbiota and CNS Development
Impact of Microbiota Composition on Neural Processes
Research in germ-free (GF) mice has provided the strongest evidence for the role of the microbiota in neurodevelopment.
Studies, where GF rodents have been re-colonized with "normal" microbiota (from specific pathogen-free animals) at different ages, have demonstrated that post-weaning re-colonization is more effective at restoring GF deficits than recolonization later in life in certain aspects of brain or immune function and behavior Buffington S.A. Di Prisco G.V. Auchtung T.A. et al. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016; 165: 1762-1775 Scopus (437) PubMed Abstract Full Text Full Text PDF Google Scholar , Clarke G. Grenham S. Scully P. et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013; 18: 666-673 Scopus (841) PubMed Crossref Google ScholarHowever, some functions in GF animals, such as those affecting CNS serotonergic neurotransmission, cannot be recovered by re-colonization by the time of weaning, indicating that the window for microbial influence on these functions has already closed.
Research has shown that the composition of the microbiota has a major impact on fundamental central neural processes such as development, myelination, neurogenesis and microglia activation Walter J. Armet A.M. Finlay B.B. et al. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell. 2020; 180: 221-232 Scopus (93) PubMed Abstract Full Text Full Text PDF Google Scholar Germ-free (GF) mice studies have provided the strongest evidence for the role of the microbiota in neurodevelopment Luczynski P. McVey Neufeld K.A. Oriach C.S. et al. Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol. 2016; 19: 1-17 Scopus (0) Crossref Google ScholarMicrobiota Influence on Cognitive Development
Post-weaning re-colonization of GF rodents with "normal" microbiota has been found to be more effective than recolonization later in life at restoring deficits seen in the GF mice when it comes to specific features of brain or immune function and behavior. Diaz Heijtz R. Wang S. Anuar F. et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011; 108: 3047-3052 Scopus (1641) PubMed Crossref Google Scholar Studies that have followed up to two years of age have repeatedly found a connection between early antibiotic exposure and cognitive development Slykerman R.F. Hood F. Wickens K. et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine. 2017; 24: 159-165 Scopus (126) PubMed Abstract Full Text Full Text PDF Google Scholar Another study found a link between cognitive ability at two years old and the composition of the microbiota a year earlier Carlson A.L. Xia K. Azcarate-Peril M.A. et al. Infant gut microbiome associated with cognitive development. Biol Psychiatry. 2018; 83: 148-159 Scopus (141) PubMed Abstract Full Text Full Text PDF Google ScholarRecently, scientists have discovered that the different types of bacteria in infants' bodies can influence their cognitive abilities when they are two years old.
What's more, they found a connection between those bacteria and the way different parts of the brain are connected.
This connection is linked to cognitive abilities at two years old Gao W. Salzwedel A.P. Carlson A.L. et al. Gut microbiome and brain functional connectivity in infants-a preliminary study focusing on the amygdala. Psychopharmacology (Berl). 2019; 236: 1641-1651 Scopus (0) PubMed Crossref Google ScholarHIGHLIGHT
Germ-free mouse research highlights microbiota's role in neurodevelopment, impacting brain functions and cognitive development, with early antibiotic exposure implications.
ENS Development
ENS Development Beyond Birth: A Lifelong Journey
Research has shown that the development of the enteric nervous system does not end after birth. In fact, it continues throughout life in animal models Roberts R.R. Murphy J.F. Young H.M. et al. Development of colonic motility in the neonatal mouse-studies using spatiotemporal maps. Am J Physiol Gastrointest Liver Physiol. 2007; 292: G930-G938 Scopus (0) PubMed Crossref Google Scholar Glial cells, which are a type of nerve cell, remain at low levels, and neurons may even be replaced more quickly than glial cells Joseph N.M. He S. Quintana E. et al. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest. 2011; 121: 3398-3411 Scopus (146) PubMed Crossref Google Scholar , Laranjeira C. Sandgren K. Kessaris N. et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest. 2011; 121: 3412-3424 Scopus (211) PubMed Crossref Google Scholar Additionally, the connections between nerve cells in the enteric nervous system may change even after adolescence Kulkarni S. Micci M.A. Leser J. et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci U S A. 2017; 114: E3709-E3718 Scopus (85) PubMed Crossref Google Scholar , Parathan P. Wang Y. Leembruggen A.J. et al. The enteric nervous system undergoes significant chemical and synaptic maturation during adolescence in mice. Dev Biol. 2020; 458: 75-87 Scopus (8) PubMed Crossref Google ScholarGut Microbiota's Impact on the Enteric Nervous System
Research into the development of the ENS has mostly looked at its molecular and genetic origins Lake J.I. Heuckeroth R.O. Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol. 2013; 305: G1-G24 Scopus (184) PubMed Crossref Google Scholar In recent years, however, there has been more focus on factors such as the gut microbiota and the immune system in the postnatal gut environment, which can help form the ENS Hyland N.P. Cryan J.F. Microbe-host interactions: influence of the gut microbiota on the enteric nervous system. Dev Biol. 2016; 417: 182-187 Scopus (71) PubMed Crossref Google ScholarStudies on GF mice (mice with a reduced number of enteric neurons and altered gut-brain neural pathways show that these mice have deficits in gut motility as well as reduced excitability of intrinsic primary afferent neurons.
However, by conventionalizing adult GF mice (giving them exposure to bacteria) the deficit in intestinal transit time can be reduced De Vadder F. Grasset E. Manneras Holm L. et al. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A. 2018; 115: 6458-6463 Scopus (113) PubMed Crossref Google Scholar , neuronal excitability can be restored Perez-Burgos A. Mao Y.K. Bienenstock J. et al. The gut-brain axis rewired: adding a functional vagal nicotinic "sensory synapse". FASEB J. 2014; 28: 3064-3074 Scopus (0) PubMed Crossref Google Scholar , enteric neuron coding can be altered and enteric glial cell density and gut physiology can be normalizedThis demonstrates that the microbiota plays an important role in ENS plasticity (the ability of the enteric nervous system to change according to the environment).
Similar effects have been noted after bacterial exposure through probiotics or specific bacterial strains Obata Y. Castano A. Boeing S. et al. Neuronal programming by microbiota regulates intestinal physiology. Nature. 2020; 578: 284-289 Scopus (53) PubMed Crossref Google Scholar Research has shown that certain microorganisms can affect the activity of the intestines' nerves Al-Nedawi K. Mian M.F. Hossain N. et al. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J. 2015; 29: 684-695 Scopus (53) PubMed Crossref Google Scholar This includes things like G-protein-coupled receptor signals, serotonin, short-chain fatty acids, interactions between microbes and the intestinal wall, and aryl hydrocarbon receptor (Ahr) , which is a type of transcription factor. Mao Y.K. Kasper D.L. Wang B. et al. Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat ommun. 2013; 4: 1465 Scopus (82) PubMed Crossref Google ScholarAryl hydrocarbon receptor (Ahr) is a type of biosensor found in the gut that helps to maintain balance between intestinal epithelial cells and immune cells.
Recently, researchers discovered that when Ahr is not present in the neurons of the gut or when its regulator (CYP1A1) is overactive, the colon muscles don't move as much as they should, similar to what happens when the gut has no bacteria Schiering C. Wincent E. Metidji A. et al. Feedback control of AHR ignalling regulates intestinal immunity. Nature. 2017; 542: 242-245 Scopus (182) PubMed Crossref Google ScholarPreclinical studies have explored the possibility of reverse regulation where the enteric nervous system influences the microbiome.
Changes in the composition of colonic and/or fecal microbiota have been observed in cases of congenital aganglionosis in animal models.
However, further research is needed to fully understand the relationship between the ENS and the microbiome Rolig A.S. Mittge E.K. Ganz J. et al. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol. 2017; 15e2000689 Scopus (65) PubMed Crossref Google ScholarHIGHLIGHT
While initial research hints at changes in microbiota composition in congenital aganglionosis animal models, further investigation is imperative to fully comprehend the complex interactions between the ENS and the microbiome.
Discussion
The gut-brain axis, a critical component in maintaining bodily homeostasis, has gained significant attention in recent years.
This axis encompasses the intricate interplay between the gut, the enteric nervous system (ENS), the central nervous system (CNS), the immune system, and the microbiome.
- Historical Context: The brain-gut connection has been recognized since ancient times, but it wasn't until the 20th century that scientists delved into how gut physiology can impact emotions and mental health.
- The Role of the Enteric Nervous System (ENS): The ENS, also known as the "second brain," is a complex neural network in the intestines. It works independently but is influenced by the CNS and autonomic nervous system. This network regulates gut function and communicates with the brain.
- Microbiota-Gut-Brain Axis: The microbiome plays a crucial role in regulating gut-brain signaling. Communication between the gut microbiota and the CNS occurs through neural, endocrine, and immune pathways, while the CNS impacts factors such as motility, immune response, and secretion. The ENS is also involved in this relationship.
- Implications for Health: The gut-brain axis is vital for our health. Disruptions in this axis can cause conditions like Parkinson's disease, and irritable bowel syndrome, and may also be linked to neurological disorders such as Alzheimer's disease, autism, and depression.
- Developmental Aspects: The gut-brain axis development is ongoing and can have long-term health implications. Factors such as antibiotic exposure, diet, stress during pregnancy, and breastfeeding can shape an individual's microbiome and impact their cognitive development.
- ENS Plasticity: The ENS is highly adaptable to environmental changes and is influenced by microbial exposure. Studies on germ-free mice have shown the crucial role of microbiota in ENS function and gut physiology.
- Mechanisms of Communication: Gut microorganisms can affect intestinal nerves through various mechanisms, including G-protein-coupled receptor signals, serotonin, short-chain fatty acids, interactions with the intestinal wall, and the aryl hydrocarbon receptor. Understanding these mechanisms is important for understanding the gut-brain connection.
- Future Therapeutic Avenues: The gut-brain axis offers new potential for therapeutic interventions. Targeting the microbiome to improve emotional well-being, cognitive function, and gastrointestinal health shows promise, but more research is required.
Conclusion
The gut-brain axis is a complex and dynamic system that plays a crucial role in maintaining overall health and well-being.
Recent research has shed light on the significant influence of the microbiome on this axis, highlighting the microbiota-gut-brain connection.
Understanding the intricacies of this communication network has the potential to revolutionize our approach to treating a range of conditions, from neurological disorders to mental health issues.
However, while current research is promising, there are still many unanswered questions, and further investigation is needed to fully harness the therapeutic potential of this axis.
Review date not set.
How we reviewed this article:
Latest on: